Israel’s IceCure granted FDA Breakthrough Medical Device designation
The decision puts the company’s tumor-freezing technology on the fast track for final approval
15:2604.04.21
Tel Aviv-traded IceCure Medical Ltd. announced on Sunday that its tumor-freezing technology ProSense has received U.S. FDA designation as a breakthrough medical device.
The new designation offers manufacturers an opportunity to interact with the FDA's experts and puts it in line for prioritized review before receiving final authorization.
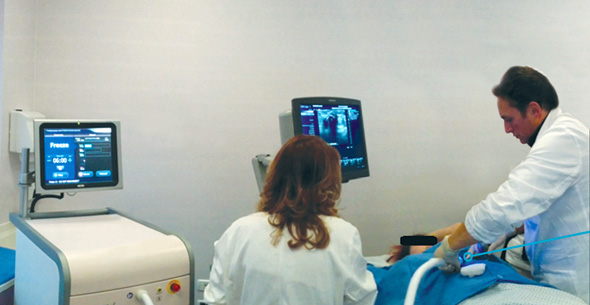
Doctors freeze a breast cancer tumor. Photo: IceCureצילום: אייסקיור
In order to receive the special designation, the FDA must determine that the device “provides for more effective treatment or diagnosis of life-threatening or irreversibly debilitating human disease or conditions” than anything currently available on the market.
Founded in 2006 and headquartered in the coastal town of Caesarea, IceCure develops an ultrasound imaging-guided probe that injects liquid nitrogen into a tumor, freezing its tissue in a process called cryoablation. The destroyed tumor cells are then reabsorbed in the body over time.
“It is estimated that 280,000 new cases of breast cancer are discovered every year in the U.S., of which ProSense is capable of treating those that were detected early on and are considered low risk, or in cases when alternative treatment is not available to the patient,” IceCure CEO Eyal Shair said. “This designation is an important milestone for the company since it offers vital regulatory recognition of the clinical need that in many cases goes unanswered when it comes to a range of cancer indications, including breast cancer.”



