Mobile Phone Urine Testing Kit Receives FDA Approval
Developed by Tel Aviv-based Healthy.io, the technology uses computer vision algorithms, artificial intelligence, and a smartphone’s built-in camera to run lab-standard urine tests
13:1226.07.18
Tel Aviv-based mobile health startup Healthy.io Ltd. has received Class II U.S. Food and Drug Administration (FDA) approval for its smartphone-based urinalysis kit, Dip.io, the company announced Wednesday.
For daily updates, subscribe to our newsletter by clicking here.
Healthy.io’s technology uses computer vision algorithms, artificial intelligence, and a smartphone’s built-in camera to perform lab-standard urine tests. The urine sample kit provided by the company includes a dipstick capable of detecting ten different parameters, including a range of infections, chronic illnesses, and pregnancy-related complications. Patients can scan the strip using their mobile phone's camera to receive instant results through an app.
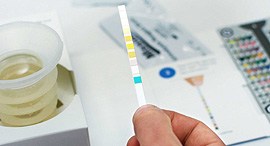
Healthy.io's urinalysis kit. Photo: PR
The FDA’s Class II approval is granted to medical and paramedical devices that have direct health implications and require a medium level of supervision. This is the first time the FDA has granted such approval for a medical device that is based on optical equipment manufactured and distributed by a third-party, Healthy.io’s founder and CEO Yonatan Adiri said in a phone interview with Calcalist on Thursday.
The FDA’s approval can serve as a precedent for other companies utilizing the ever improving camera technologies available on mobile phones to create accessible household medical devices for patients, Adiri said.
Founded in 2013, Healthy.io has raised $15 million to date from Quantum Pacific International Ltd., a company owned by Israeli businessman Idan Ofer. The company has around forty employees in offices in Tel Aviv, London, and New York.
Related articles
In April, Healthy.io announced a partnership with the American National Kidney Foundation and Pennsylvania-based Geisinger Health System to launch a clinical trial of the technology among Geisinger’s patients.
Healthy.io’s product has already been approved by health agencies in Israel and the U.K., and the company aims to reach 100,000 active users by the end of this year, Healthy.io said in a statement.



